Study: Smoke particles from wildfires can erode the ozone layer
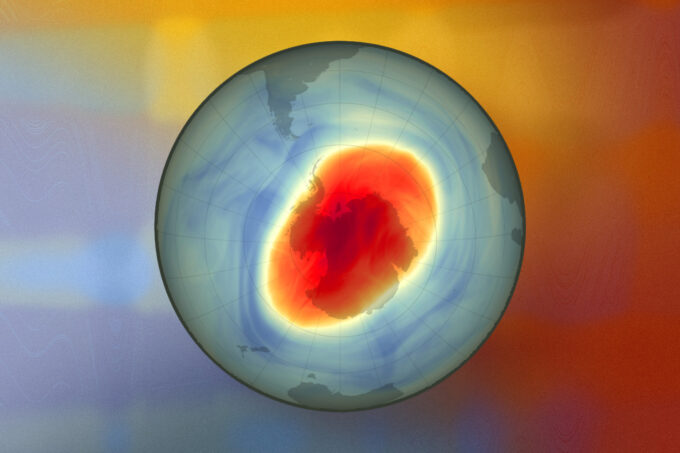
An MIT study finds that smoke particles in the stratosphere can trigger chemical reactions that erode the ozone layer — and that smoke particles from Australian wildfires widened the ozone hole by 10 percent in 2020. This map shows the size and shape of the ozone hole over the South Pole on Oct. 5, 2022. Image credit: NASA Earth Observatory image by Joshua Stevens. Edited by MIT News.
A wildfire can pump smoke up into the stratosphere, where the particles drift for over a year. A new MIT study has found that while suspended there, these particles can trigger chemical reactions that erode the protective ozone layer shielding the Earth from the sun’s damaging ultraviolet radiation.
The study, which appears today in Nature, focuses on the smoke from the “Black Summer” megafire in eastern Australia, which burned from December 2019 into January 2020. The fires — the country’s most devastating on record — scorched tens of millions of acres and pumped more than 1 million tons of smoke into the atmosphere.
The MIT team identified a new chemical reaction by which smoke particles from the Australian wildfires made ozone depletion worse. By triggering this reaction, the fires likely contributed to a 3-5 percent depletion of total ozone at mid-latitudes in the Southern Hemisphere, in regions overlying Australia, New Zealand, and parts of Africa and South America.
The researchers’ model also indicates the fires had an effect in the polar regions, eating away at the edges of the ozone hole over Antarctica. By late 2020, smoke particles from the Australian wildfires widened the Antarctic ozone hole by 2.5 million square kilometers — 10 percent of its area compared to the previous year.
It’s unclear what long-term effect wildfires will have on ozone recovery. The United Nations recently reported that the ozone hole, and ozone depletion around the world, is on a recovery track, thanks to a sustained international effort to phase out ozone-depleting chemicals. But the MIT study suggests that as long as these chemicals persist in the atmosphere, large fires could spark a reaction that temporarily depletes ozone.
“The Australian fires of 2020 were really a wake-up call for the science community,” says Susan Solomon, the Lee and Geraldine Martin Professor of Environmental Studies at MIT and a leading climate scientist who first identified the chemicals responsible for the Antarctic ozone hole. “The effect of wildfires was not previously accounted for in [projections of] ozone recovery. And I think that effect may depend on whether fires become more frequent and intense as the planet warms.”
The study is led by Solomon and MIT research scientist Kane Stone, along with collaborators from the Institute for Environmental and Climate Research in Guangzhou, China; the U.S. National Oceanic and Atmospheric Administration; the U.S. National Center for Atmospheric Research; and Colorado State University.
Chlorine cascade
The new study expands on a 2022 discovery by Solomon and her colleagues, in which they first identified a chemical link between wildfires and ozone depletion. The researchers found that chlorine-containing compounds, originally emitted by factories in the form of chlorofluorocarbons (CFCs), could react with the surface of fire aerosols. This interaction, they found, set off a chemical cascade that produced chlorine monoxide — the ultimate ozone-depleting molecule. Their results showed that the Australian wildfires likely depleted ozone through this newly identified chemical reaction.
“But that didn’t explain all the changes that were observed in the stratosphere,” Solomon says. “There was a whole bunch of chlorine-related chemistry that was totally out of whack.”
In the new study, the team took a closer look at the composition of molecules in the stratosphere following the Australian wildfires. They combed through three independent sets of satellite data and observed that in the months following the fires, concentrations of hydrochloric acid dropped significantly at mid-latitudes, while chlorine monoxide spiked.
Hydrochloric acid (HCl) is present in the stratosphere as CFCs break down naturally over time. As long as chlorine is bound in the form of HCl, it doesn’t have a chance to destroy ozone. But if HCl breaks apart, chlorine can react with oxygen to form ozone-depleting chlorine monoxide.
In the polar regions, HCl can break apart when it interacts with the surface of cloud particles at frigid temperatures of about 155 kelvins. However, this reaction was not expected to occur at mid-latitudes, where temperatures are much warmer.
“The fact that HCl at mid-latitudes dropped by this unprecedented amount was to me kind of a danger signal,” Solomon says.
She wondered: What if HCl could also interact with smoke particles, at warmer temperatures and in a way that released chlorine to destroy ozone? If such a reaction was possible, it would explain the imbalance of molecules and much of the ozone depletion observed following the Australian wildfires.
Smoky drift
Solomon and her colleagues dug through the chemical literature to see what sort of organic molecules could react with HCl at warmer temperatures to break it apart.
“Lo and behold, I learned that HCl is extremely soluble in a whole broad range of organic species,” Solomon says. “It likes to glom on to lots of compounds.”
The question then, was whether the Australian wildfires released any of those compounds that could have triggered HCl’s breakup and any subsequent depletion of ozone. When the team looked at the composition of smoke particles in the first days after the fires, the picture was anything but clear.
“I looked at that stuff and threw up my hands and thought, there’s so much stuff in there, how am I ever going to figure this out?” Solomon recalls. “But then I realized it had actually taken some weeks before you saw the HCl drop, so you really need to look at the data on aged wildfire particles.”
When the team expanded their search, they found that smoke particles persisted over months, circulating in the stratosphere at mid-latitudes, in the same regions and times when concentrations of HCl dropped.
“It’s the aged smoke particles that really take up a lot of the HCl,” Solomon says. “And then you get, amazingly, the same reactions that you get in the ozone hole, but over mid-latitudes, at much warmer temperatures.”
When the team incorporated this new chemical reaction into a model of atmospheric chemistry, and simulated the conditions of the Australian wildfires, they observed a 5 percent depletion of ozone throughout the stratosphere at mid-latitudes, and a 10 percent widening of the ozone hole over Antarctica.
The reaction with HCl is likely the main pathway by which wildfires can deplete ozone. But Solomon guesses there may be other chlorine-containing compounds drifting in the stratosphere, that wildfires could unlock.
“There’s now sort of a race against time,” Solomon says. “Hopefully, chlorine-containing compounds will have been destroyed, before the frequency of fires increases with climate change. This is all the more reason to be vigilant about global warming and these chlorine-containing compounds.”
This research was supported, in part, by NASA and the U.S. National Science Foundation.